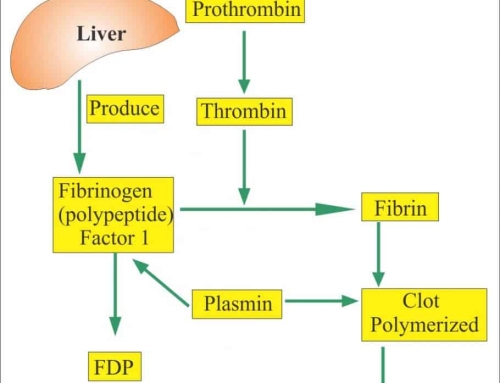
In the diagnosis of acute infection of pathogens, igm antibodies are usually required, such as serum anti-havigm detection of acute hepatitis a diagnosis, serum anti-hbclgm detection of acute hepatitis b virus infection and series igm detection of torch project, etc. IgM antibodies are also measured using indirect methods, such as some of the IgM detection kits of the torch series currently available on the market. In the indirect method of IgM antibody, because the clinical serum sample contains high concentration of IgG antibody, some of the specific IgG antibody will compete with IgM antibody and bind to solid phase antigen. disturbing the detection of igm antibodies.
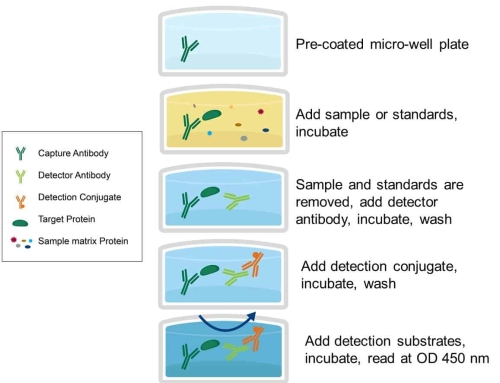
Therefore, in the use of indirect assays for the determination of IgM antibodies, serum samples are usually subject to pretreatment with anti-human IgG antibodies or SPA to remove IgG interference. This not only tedious determination, but also affect the specificity and sensitivity of the determination. At present, the commonly used detection method of IgM antibody is capture method, that is, using anti-human IgM antibody (anti-human u-chain) as solid-phase antibody, when adding serum samples, IgM class antibody (specific and non-specific) can be captured by solid-phase antibody, and then add specific antigen, which binds to IgM antibody captured on solid-phase. after that, the antibody of enzyme labeled anti-specific antigen was added, and finally the substrate color was added. The operational steps are as follows:
1. Firstly, the anti-human IgMbt chain antibody was coated overnight with solid phase such as polystyrene in carbonate buffer solution for 40c to form solid phase antibody. After washing and removing the unconjugated or unbonded antibody, it was closed with calf serum or bovine serum albumin to remove unconjugated parts and impurities.
2. Add clinical samples with IgM antibody to be tested, such as serum, after incubation for a certain period of time to wash the plate; at this time, the IgM antibody in the sample to be tested will be with the anti-P chain on the solid phase. the antibody reacts while adsorbing on the solid phase.
3. Add specific antigens such as HAV antigen, HBcAg, etc., after incubation for a certain period of time, wash the plate; at this time, the specific antigen will react with specific IgM antibodies on the solid phase.
4. Add the enzyme-labeled anti-specific antigen antibody, after incubation for a certain period of time after washing the plate; at this time, the corresponding antigen-antibody complex is formed on the solid phase.
5. Determination of color by adding enzyme substrate
this method should focus on the interference of rf (class 1gm) and other non-specific igm. RF (1gM class) as a result which can bind to solid-phase anti-human u-chain antibodies and can react with subsequently added enzyme-labeled antibodies (animal iggg), leading to false positive reactions. whereas nonspecific igm can affect the sensitivity of the assay because it can compete with specific igm in the first step of incubation and bind to solid-phase antibodies. Therefore, using this method to measure IgM, clinical samples must be properly diluted. After dilution of the sample, the non-specific IgM content of the above-mentioned interferences decreased, while the specific IgM had no significant effect after certain dilution due to its high titer during the acute infection period of the corresponding pathogen, moreover, in some pathogens such as HBV In chronic infection, IgM-specific antibodies persist, except for a much lower titer.
Therefore, if the serum sample is not diluted, it is detected directly, even without the interference of nonspecific IgM, and the positive test results have no diagnostic value of acute infection. Nowadays, some reagent manufacturers, in order to meet the requirements of clinical laboratory to reduce labor intensity and simple operation, have produced anti-HAVIgM and anti-HBclgM ELISA kits that do not need to dilute the samples, and many existing laboratories are also in use. For the reasons mentioned above, we recommend that the clinical laboratory do Classes such as anti-HAVIGM and anti-HBclgM should be tested using a dilution kit to ensure the clinical value of the test.
Common abbreviations for determination of ELISA results by enzyme-linked immunosorbent assay
ELISA is divided into qualitative and quantitative determinations according to the way it represents the results. The qualitative determination is only to make the conclusion of whether the specimen contains the antigen or antibody to be tested “have” or “no “, expressed as” positive “and” negative “respectively.
Visible qualitative determinations are usually antigens or antibodies used in infectious pathogens the determination of the body to determine the presence or absence of a specific pathogen infection. Quantitative determination is to measure the number of antigens to be tested in the specimen, expressed as specific values. quantitative determination is basically used for the determination of non-pathogen antigenic substances, such as hormones, cytokines, tumor markers, small molecule drugs, etc. At present, most of the ELISA kits used in China are used for the qualitative determination of antigens or antibodies of infectious pathogens.
quantitative determination of fp, hcg, cytokines, etc. The determination of “negative” and “positive” by ELISA was based on reagents positive determination value determined by the box (cut-off). The quantitative determination of “values” is based on the dose response curve (also called the standard curve) obtained from the simultaneous determination of the standard product in the kit.
laboratory for indoor quality control use.
Here are some of the abbreviations commonly used in determining the qualitative results of ELISA:
(1) S/CO: where S is the abbreviation of sample (sample) or specmen (specimen), indicating the absorbance value determined by the specimen, and CO is the abbreviation of cut-off value 。 In addition to the competition inhibition method, in other ELISA qualitative determination modes, when the S/CO value is greater than or equal to 1, the determination of the specimen is positive and less than 1 is negative.
(2) S/N or P/N: where S is the same as (1), N is the abbreviation of negative (negative control) and P is the abbreviation of patient (patient). Many of the earlier kits used S/N or P/N ≥2.1 as positive criteria, and some still do. There is no fundamental difference between this approach and the S/CO approach, except that the former would negatively control (N)2. Double the cut-off value.
Key operational points for enzyme-linked immunosorbent assay
1. Adoption and preservation of specimens
There are a wide range of specimens that can be used for ELISA determination, including body fluids (e.g. serum), secretions (saliva) and excreta (e.g. urine, feces). Some specimens can be determined directly (e.g. serum, urine), while others require pretreatment (e.g. feces and certain secretions). most of the elisa assays were serum samples. The plasma contains fibrinogen and anticoagulants, all of which are equal to serum. The preparation of plasma samples requires the aid of anticoagulant, while serum samples need to be naturally coagulated and blooded after the block shrinks can be obtained. Except for special cases, serum is used as test specimen in medical examination. plasma and serum can be applied equally in ELISA. Serum samples can be collected according to the conventional method, should pay attention to avoid hemolysis, red blood cell dissolution will release a substance with peroxidase activity, in the ELISA assay marked with HRP, hemolytic samples may increase non-specific color rendering.
Serum samples should be tested when fresh. If there is bacterial contamination, the bacteria may contain endogenous HRP, will also produce false positive reaction. If stored in the refrigerator for too long, the polymerization may occur indirectly ELISA can deepen the background. in general, serum specimens measured within 5 days can be placed at 4°c, more than a week of determination required for cryogenic ice storage. After freezing the serum melt, the protein is concentrated locally, the distribution is uneven, it should be fully mixed gently, to avoid bubbles, can be reversed up and down mixing, do not strongly oscillate on the mixer. Serum specimens with turbidity or precipitation should be centrifuged or filtered before detection. Repeated freezing and thawing will make the antibody titer drop, so if the serum samples of the antibody need to be stored for multiple tests, a small amount of ice storage. Keep serum self-collection should pay attention to aseptic operation, also can add appropriate preservative.
2.Preparation of reagents
Prepare the reagents required in the experiment as required by the kit instructions. distilled or deionized water used in elisa, including for washing, shall be fresh and of high quality. the self-matched buffer was positively measured using a ph meter. The test reagents removed from the refrigerator should be used after the temperature is balanced with room temperature. The unused parts of the kit should be stored in the refrigerator in time.
3 Sample
in elisa, there are generally 3 times to add sample step polymerization, that is, add specimen, add enzyme conjugate, add substrate. Additions should be added Add to the bottom of the LEISA plate hole, avoid adding to the upper part of the hole wall, and pay attention not to splash out, not to produce bubbles.
The specimen is generally added with a microsampler and added to the plate hole according to the prescribed quantity. Each time the standard should be replaced suction nozzle to avoid cross contamination, but also can be a one-time quantitative plastic pipe sample. This determination (e.g. indirect ELISA) requires the use of diluted serum, which can be diluted in the test tube according to the prescribed dilution before adding samples. The dilutions can also be added to the plate hole, then added to the serum specimen, and then shocked on the micro-concert for 1 minute to ensure mixing. Application of enzyme conjugate and substrate quantitative multi-channel liquid-adder, so that the liquid-adding process can be completed quickly.
4 Insulation
There are generally two antigen-antibody reactions in ELISA, that is, after adding the specimen and the enzyme conjugate. the completion of the antigen-antibody reaction requires a certain temperature and time. this holding process is called incubation, which some call incubation, and seems inappropriate in elisa.
ELISA is a solid-phase immunoassay, and the binding of antigens and antibodies only occurs on the solid surface. Taking the sandwich method of the antibody coating as an example, the specimens in the plate hole were not all antigens Equal and solid-phase anti-binding opportunities, only the closest to the pore wall in a layer of solution of antigen directly contact with the antibody. This is a gradually equilibrium process and therefore requires diffusion to reach the end point of the reaction. The same is true for the binding of enzyme-labeled antibodies to solid-phase antigens that are subsequently added. this is why the elisa reaction always requires a certain amount of incubation time.
The temperatures commonly used in warming education are 43°C,37°C, room temperature and 4°C (refrigerator temperature), etc. 37°C is the commonly used holding temperature in the laboratory, and it is also the appropriate temperature for most antigen-antibody binding. In establishing ELISA method for reaction kinetics At 37°C for 1-2 hours, the production of the product peaked. In order to accelerate the reaction, the temperature of the reaction can be increased, some tests are carried out at 43°C, but it is not suitable to use higher temperature. The antigen-antibody reaction was more thorough at 4°C, and the reaction was mostly overnight in the refrigerator to form the most precipitate in the radioimmunoassay. However, due to the long time required, it is generally not used in ELISA.
In addition to some ELISA instruments with special electric heating block, generally use water bath, ELISA board can be placed in the water bath box, ELISA A The bottom of the plate should be attached to the water to make the temperature balance quickly. To avoid evaporation, the plate should be capped, can also be covered with plastic sealing paper or cling film plate hole, at this time can let the reaction plate floating on the water. If use the incubator, the ELISA board should be placed in the wet box, the wet box should choose good heat transfer materials such as metal, in the box bottom pad wet gauze, finally the ELISA board on the wet gauze. The wet box should be pre-warmed in the incubator to the specified temperature, especially when the temperature is low. Whether it is water bath or wet box heating, the reaction plate should not be stacked to ensure that the temperature of each plate can be quickly balanced. Response to room temperature incubation The operating room temperature shall be strictly limited to the prescribed range.The standard room temperature refers to 20-25°C, but the specific operation may be controlled according to the requirements of the instructions. At room temperature, the ELISA plate can be laid flat on the operating table. Attention should be paid to the temperature and time of incubation should strive for accuracy according to the regulations. To ensure this, one should not operate more than two plates at a time.
5 Washing
Although washing is not a reaction step in the elisa process, it also determines the success or failure of the experiment. ELSIA is by washing to achieve separation of free and combined the purpose of the enzyme marker. by washing to remove the remaining substances that did not bind to the solid antigen or antibody in the plate pore and the interfering substances that were nonspecifically adsorbed to the solid carrier during the reaction. The adsorption of protein by polystyrene and other plastics is universal, and the non-specific adsorption interference material should be washed down when washing. It can be said that in ELISA operation, washing is the most important key technology, which should cause the operator to attach great importance to it, and the operator should wash it strictly according to the requirements and not be careless.
Washing methods except that some ELISA instruments are equipped with special automatic scrubbers There are two types: soaking and water washing. The process is as follows:
(1)Immersion
a. dry or dump the reaction fluid in the hole;
b. Overwash with the washing liquid (after filling the plate hole with the washing liquid, shake it off);
c. Soak, that is, the washing liquid fill the plate hole, put 1-2 minutes, intermittent shaking, soaking time cannot be shortened at will;
d. Dry the liquid in the hole. Drying should be thorough, can be pump or vacuum pump suction, can also be dumped after the liquid on a clean towel or absorbent paper pat dry;
e. repeat operations c and d, wash 3-4 times (or as per instructions). In the indirect method, if the background is higher, the washing times can be increased or extended soaking time.
The micro titration plate adopts soaking washing method. The washing liquid is mostly neutral buffer containing non-ionic detergent. the binding of polystyrene carrier to protein is hydrophobic. the non-ionic detergent contains both hydrophobic group and hydrophilic group. its hydrophobic group binds to the hydrophobic group of protein by hydrophobic bond, thus weakening the binding of protein to solid-phase carrier, and by means of the binding of hydrophilic group and water molecule, the protein is restored to aqueous solution state and thus detached from solid-phase carrier. The non-ionic detergent in the detergent is usually Tween 20, with concentrations ranging from 0.05% to 0.2% higher than 0.2% can reduce the sensitivity of the test by desorbing the antigen or antibody coated on the solid phase.
(2) The water washing method was originally used for the washing of small bead carriers. The washing liquid is only distilled water or even tap water. A special device is attached to the washing, so that the small beads are continuously rolled and washed under the impact of running water, after continuous washing for 2 minutes, dry the liquid, then soak in distilled water for 2 minutes, and dry it dry. Soaking is like a bath, water washing is like a shower, its washing effect is more thorough, but also simple and fast. Experiments have shown that water washing is also suitable for trace amounts washing of the titration plate. When washing, try to increase the water flow or increase the water pressure, so that the water flow impact on the surface of the plate hole, washing effect is better.
ELISA Testing — Competition Method for Antibodies
antibody determination generally does not use the competition method. When the impurities in the antigen are difficult to remove or the binding specificity of the antigen is unstable, the antibody can be determined in this mode. For example, the determination of HBV core antibody (HBcAb) and HBV e antibody (HBeAb), because e antigen is only 29 amino acids more than core antigen, e antigen is easily transformed into core antigen, because Therefore, both HBcAb and HBeAb were determined by competition method. But there are differences in its specific patterns. The solid phase of antigen can be either direct or indirect solid phase by corresponding specific antibody.
The following are the specific operational steps for the determination of HBcAb and HBeAb ELISA as follows.
1. Determination of HBcAb by Competition Law
(1) The HBcAg was first coated overnight with 4~C in carbonate buffer to form a solid antigen. After washing and removing the unconjugated or unbonded antibodies, it was closed and washed with calf serum or bovine serum albumin Remove unconjugated parts and impurities.
(2) Add the specific antibodies of the sample to be tested and the enzyme label at the same time, and then wash the plate after incubation for a certain period of time; in this step, the antibodies of the sample to be tested will compete with the enzyme labeled antibodies to bind to the solid specific antigen.
(3) The content of the corresponding antibody in the sample to be tested is inversely proportional to the color intensity of the enzyme substrate.
2. Determination of HBeAb’s Competition Law
(1) The HBeAb was first coated overnight at 4°C in carbonate buffer to form a solid antibody, and then washed to remove antigens that did not bind or bind tightly to solid phase , seal with calf serum or bovine serum albumin, wash and remove unconjugated parts and impurities;
(2) adding both the sample to be tested and the neutralizing antigen HBeAg to warm up the plate after washing for a certain period of time; in this step, the antibody of the sample to be tested will compete with the solid antibody to bind to the neutralizing antigen HBeAg, and the higher the concentration of HBeAb in the sample to be tested, the less HBeAg will bind to the solid phase HBe-Ab, and vice versa;
(3) Adding specific antibodies to the enzyme label and incubating the plate after washing for a certain period of time; (4) The content of the corresponding antibody in the sample to be tested is inversely proportional to the color intensity of the enzyme substrate.
The reason why HBeAb is to be determined in this mode is mainly due to the instability of HBeAg, such as the direct coating of HBeAg in solid phase, which will lead to the error of determination because of the easy transmutation of HBeAg to HBcAg.
The competition method for the determination of antibodies is different from the competition method for the determination of small molecular antigens with only a single antigen-determining cluster. The reliability of the determination is greatly influenced by the specificity and affinity of competitive antibodies. The closer the specificity and affinity of the antibody and the antibody to be tested are, the more reliable the determination is, but the competition antibody is obtained by the corresponding antigen and immune animal, and the antibody produced by the body after infection with the virus is certainly different.
ELISA Testing — Competition Method for Antigen
It has more than two antigenic determinants, and can be measured by double antibody sandwich method Because they have only one antigenic determinant, they cannot be determined by double-antis Sandwich method and can only be determined by competitive inhibition method.
The steps are as follows:
1. The specific antibodies against small molecules, such as the double antibody sandwich method, were first coated and sealed;
2. Add the small molecule of the sample to be tested and the enzyme label to warm up the plate after a certain period of time; in this step, the small molecule of the sample to be tested will compete with the enzyme labeled small molecule and bind to the specific antibody on the solid phase;
Add enzyme substrate, warm The color intensity is inversely proportional to the content of small molecules in the sample to be tested.
In this determination mode, it is necessary to obtain the conjugate of small molecule and enzyme, and the small molecule enzyme conjugate one is not as simple as the antibody enzyme conjugate in preparation, the other is difficult to purify, and it is difficult to separate the enzyme with small molecule from the free enzyme. therefore, some attempts have been made to establish a biantis sandwich competitive elisa method for the determination of small molecules on the basis of the synthesis of di or polymeric small molecules, and the sensitivity and specificity of the determination have been improved.
ELISA Testing — Competition Method for Antibodies
Antibody determination generally does not use the competition method. When the impurities in the antigen are difficult to remove or the binding specificity of the antigen is unstable, the antibody can be determined in this mode. such as the determination of hepatitis b virus core antibody (hbcab) and hepatitis b virus e antibody (hbe•ab). since the e antigen has only 29 more amino acids than the core antigen, the e antigen is easily transformed into the core antigen. therefore, both hbcab and hbab are determined by competitive method. But there are differences in its specific patterns. The solid phase of antigen can be directly It can also be indirectly immobilized by corresponding specific antibodies.
The following are the specific operational steps for the determination of HBcAb and HBeAb ELISA as follows.
1. Determination of HBcAb by Competition Law
(1) The HBcAg was first coated overnight in carbonate buffer solution to form a solid antigen. After washing and removing the unconjugated or unconjugated antibodies, it was closed with calf serum or bovine serum albumin to remove the unconjugated parts and impurities.
(2) Add the specific antibody to the sample and enzyme label at the same time, and then wash it after incubation for a certain period of time plate; in this step, the antibody of the sample to be tested will compete with the enzyme labeled antibody to bind to the specific antigen on the solid phase.
(3) The content of the corresponding antibody in the sample to be tested is inversely proportional to the color intensity of the enzyme substrate.
2. Determination of HBeAb’s Competition Law
(1) The HBeAb was first coated overnight at 4°C in carbonate buffer to form a solid antibody. After washing and removing antigens that did not bind to solid phase or were not tightly bound, it was closed with calf serum or bovine serum albumin to remove unconjugated parts and impurities.
(2) At the same time In this step, the antibody of the sample will be competitively combined with the solid antibody to neutralize the antigen HBeAg, and the higher the concentration of HBeAb in the sample to be tested, the less HBeAg will bind to the solid phase HBe-Ab, and vice versa.
(3) Adding specific antibodies to the enzyme label and incubating the plate after washing for a certain period of time;
(4) The content of the corresponding antibody in the sample to be tested is inversely proportional to the color intensity of the enzyme substrate. The reason why HBeAb is to be determined in this mode is mainly due to the instability of HBeAg, such as the direct coating of HBeAg in solid phase, which will lead to the error of determination because of the easy transmutation of HBeAg to HBcAg.
The competition method of antibody determination is different from the competition method of small molecular antigen with only single antigen-determining cluster. The reliability of the determination is greatly influenced by the specificity and affinity of the competition antibody. The closer the specificity and affinity of the competition antibody and the antibody to be tested are, the stronger the reliability of the determination is. Immune animals obtained, and the body after the infection of the virus produced antibodies will certainly be different, therefore, in the current clinical detection of HBeAb and HBcAb, there are often difficult to explain the results of the determination, which is inseparable from its inherent defects in methodology.
ELISA Testing — Competition Method for Antigen
Because there are more than two antigenic determinants in macromolecules, they can be determined by double antibody sandwich method. Small molecule semi-antibodies such as digoxin, theophylline and T3, T4 and testosterone cannot be determined by double antibody sandwich method.
The steps are as follows:
1. The specific antibodies against small molecules, such as the double antibody sandwich method, were first coated and sealed;
2. Add the small molecule of the sample to be tested and the enzyme label to warm up In this step, the small molecule of the sample to be tested will compete with the enzyme labeled small molecule and bind to the specific antibody in solid phase.
3. Add the enzyme substrate, warm color determination, the color intensity of the sample is inversely proportional to the content of small molecules.
In this determination mode, it is necessary to obtain the conjugate of small molecule and enzyme, and the small molecule enzyme conjugate one is not as simple as the antibody enzyme conjugate in preparation, the other is difficult to purify, and it is difficult to separate the enzyme with small molecule from the free enzyme. Therefore, it has been attempted to build a double-antis Sandwich competitive ELISA based on the synthesis of di- or poly-small molecules The sensitivity and specificity of A method were improved.
ELISA Testing — Indirect Determination of Antibodies
The basic methods are as follows:
1. The antigens of the pathogens were coated overnight in carbonate buffer solution to form solid antigens. After washing and removing antigens that did not bind or were not tightly bound, the antigens were closed with calf serum or bovine serum albumin, and the unconjugated parts and impurities were washed and removed;
Add clinical samples containing antibody to be tested, such as serum, to warm up certain time After washing the plate, the antibody to be tested will be adsorbed on the solid phase by reacting with the specific antigen.
3. Add the enzyme-labeled anti-human IgG antibody, after incubation for a certain period of time, wash the plate; at this time, the solid phase antigen-antibody-enzyme-labeled second antibody complex is formed on the solid phase;
4. The enzyme substrate was added.
The most commonly used indirect antibodies are hepatitis C virus antibody (anti-HCV), human immunodeficiency virus antibody (anti-HIV) and Treponema pallidum antibody. It can be seen from the above determination pattern that the antibody is measured indirectly and strictly To speak, the determined IgG class is only antibody and does not involve IgM and IgA class, which is determined by the enzyme labeled II antibody.
A major factor affecting the indirect method for antibody determination is the purity of the coating antigen. now the antigens used in indirect antibodies are generally genetically engineered recombinant antigens, such as hcv ’ s ns3, ns4, ns5, hiv ’ s gp41 and gpl20 and syphilis spirochetes tpnl5, tpnl7, tpn47, etc. Genetically engineered antigens should be purified by removing the antigens used to express the host, such as E. coli, so as not to be affected by the presence of the body Antibodies to host antigens cause false positive reactions. in addition, because of the high concentration of iggg antibodies in the body, most of them are non-specific igg produced by the body’s exposure to external environmental stimuli. therefore, to avoid the false positive reaction caused by the adsorption of these high concentrations of non-specific igg to solid phase, it is usually necessary to treat the measured samples to a certain extent of dilution.
Clinical ELISA Determination of Common Pattern-Double antibody sandwich method for antigen
For macromolecular proteins with multiple antigenic determinants, the double antibody sandwich ELISA pattern is fairly simple Thus, the existing commodity kits are largely in this mode of determination. The methods are as follows:
1. Firstly, the solid phase such as polystyrene was coated overnight with one of the double antibodies in carbonate buffer at 4°C to form a solid antibody. After washing and removing the unconjugated or unbonded antibodies, the unconjugated parts and impurities were closed with calf serum or bovine serum albumin.
2. To add clinical samples containing the substance to be tested, such as serum, after incubation for a certain period of time, wash the plate; at this time, the antigen to be tested will react with specific antibodies on the solid phase and adsorb on the solid phase;
3. Adding enzymes Two of the marked double antibodies, after incubation for a certain period of time after washing the plate; at this time, the formation of double antibody and specific antigen on the solid phase of the sandwich product;
4. The enzyme substrate was added.
In this assay mode, there are two steps of incubation and plate-hole washing steps. if the above assay steps 2 and 3 are combined in one step, the sample to be tested and the enzyme-labeled antibody are added at the same time, thus there is only one step of incubation and plate washing process, which is commonly referred to as “one-step method “. The two-step method was used for the initial double antibody sandwich ELISA kit. Later, in order to save time and simplify the operation, people tried The one-step kit was introduced step by step by the manufacturer. currently in our clinical laboratory for the determination of macromolecular antigens such as hbsag,. fp and hCG, etc., basically all adopt one-step method. the one-step method compared to the two-step method, although the operation is simple’ but has its inherent defects, and the treatment is not good, which has a serious impact on the results of elisa determination.
In the usual two-step incubation and washing method, the antigen-antibody reaction will follow the following rule: when the concentration of antigen (Ag) in the specimen to be tested is gradually increased in the first step, the binding of solid-phase antibody (Ab) to antigen will gradually reach saturation [(1)] formula], so that when a certain concentration of enzyme-labeled antibody (Ab’) is subsequently added, the formation of a complex will be directly related to the b complex formed in the first step [(2)]. Therefore, the gradual increase of the antigen concentration to be tested leads to the gradual deepening of the color development, and when the antigen concentration increases to a certain extent, the reaction reaches the platform and presents a S-shaped change curve.
The reaction curve of one-step double antibody sandwich ELISA was bell-shaped. That is to say, the determination of color with the increase of antigen concentration in the sample increases to a certain extent, the determination of absorbance with the increase of antigen concentration The so-called hook effect, or zonephen, is what we call zonephen in immunoprecipitation tests. menon). One-step reaction equilibrium formulae such as (3)
In addition, the increase of b and c complex also makes it difficult to form “double antibody sandwich complex “. However, if solid-phase monoclonal antibody and marker monoclonal antibody use antibodies against different and distant determinants of antigen, that is, coated with one monoclonal antibody, enzyme labeling uses another monoclonal antibody, while fully mixing the reaction solution and appropriately prolonging the reaction temperature time, then can make b,. and d complex reduced to a minimum, while significantly reducing the “hook-like effect ”.
We used the serum samples of HBsAg containing the highest concentration (about 2 mg/m1) available in this chamber to evaluate the “one-step” HBsAg determination of the “hook effect” of the ELISA kit by the larger domestic manufacturers of reagents. Although most of the samples were tested at the highest concentration of HBsAg (1 lo dilution), they were not found to be shallow. Still, in clinical practice, it is still possible to encounter a “hook-like effect ” more severe hbsag assay elisa kit, and we also often hear responses from our grassroots laboratory counterparts in this regard. Therefore, we should also pay attention to this aspect of the problem •, when it is found that the test results are obviously inconsistent with the clinical or with the relevant measurement indicators, such as HBeAg positive sample HBsAg measurement is negative, we should consider the possible “hook effect” of HBsAg measurement.
To sum up, the one-step ELISA kit, as a product to meet the simple and rapid requirements of the clinical laboratory, has a huge market, although it has potential In the defect, it is easy to cause the high concentration of specimens to be tested negative (false negative), but careful experimental design, careful selection of coating and enzyme-labeled antibodies, can completely minimize the possibility of “hook effect “. in addition, it is recommended that the manufacturer of hbsag, afp and hcgelisa “one-step ” assay kits should fully study the“ hook effect ” of the produced reagents before they leave the factory, and remind the user that attention should be paid when using them.
Another important part of the double antibody sandwich test for antigen is rheumatoid factor (RF) The interference. we know that rf is an autoantibody against denaturing iggg, mainly igm antibodies of 19s, and also igg and iga antibodies of 7s can be seen. This autoantibody is characterized by its ability to bind to the Fc portion of the denatured IgG in multiple animals. therefore, if the serum specimen contains rf, it can be used as a bridging antigen between the solid-phase antibody and the enzyme-labeled antibody (both igg) for detection of the double antibody sandwich elisa, resulting in a false positive reaction. therefore, the interference of rf can be avoided if the antibody’s fab2: or fab fragment is used as an antibody for enzyme labeling.