Principle ——Biotin-Avidin System
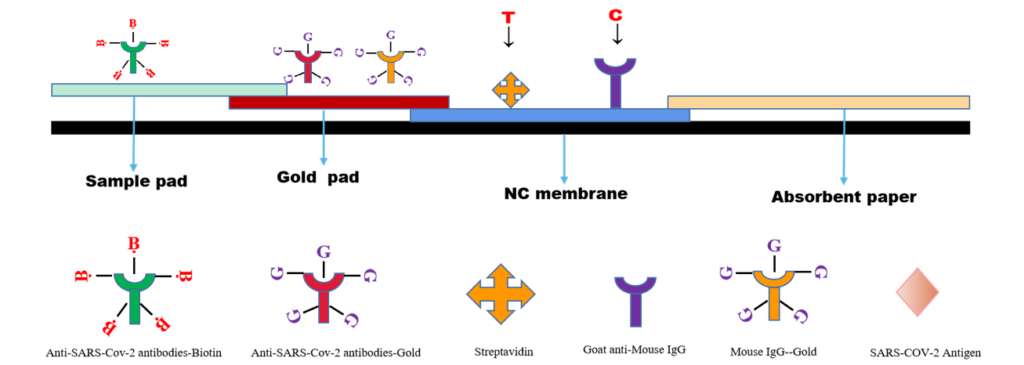
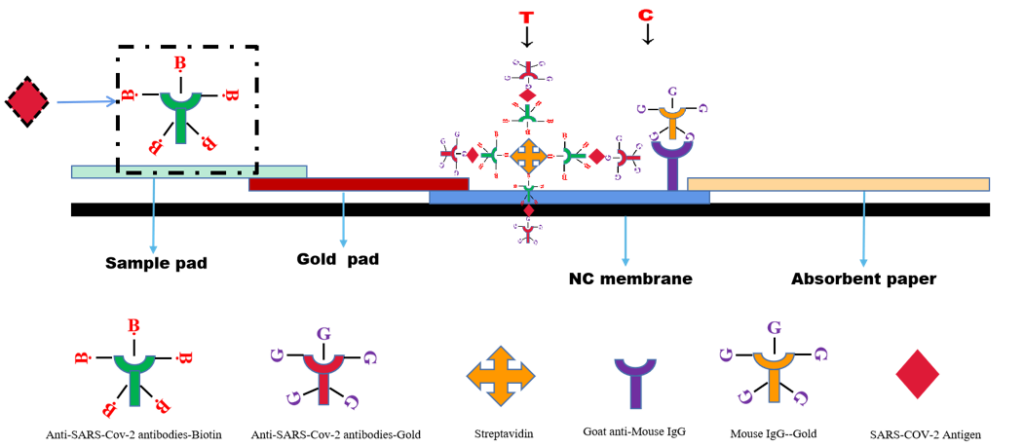
The SARS CoV-2 Antigen Rapid Test(Saliva Test) is a lateral flow chromatographic immunoassay. The test cassette consists of: 1)a burgundy colored conjugate pad containing Mouse anti-SARS-CoV-2 antibody conjugated with colloid gold (SARS CoV-2 conjugates) and mouse IgG-gold conjugates, 2) a nitrocellulose membrane strip containing two test bands (test band) and a control band (C band). The test band is pre-coated with Streptavidin .The C band is pre-coated with goat anti mouse IgG.3)the sample pad is precoated with Mouse anti-SARS-CoV-2 antibody conjugated with Biotin.
When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. SARS-CoV-2 virus if present in the specimen will bind to the monoclonal mouse anti- SARS-CoV-2 antibody on the sample pad.The immunocomplex is then captured on the gold pad by the pre-conjugated mouse anti-SARS-CoV-2 antibody. With chromatography, the immunocomplex is captured on the membrane by the pre-coated streptavidin.Streptavidin will conjugated with biotin- SARS-COV-2 Antibody,and forming a burgundy colored T band, indicating a Covid-19 antigen positive test result. Absence of test band (T) suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti rabbit IgG/rabbit IgG-gold conjugate regardless of the color development on any of the test bands. Otherwise, the test result is invalid, and the specimen must be retested with another device.
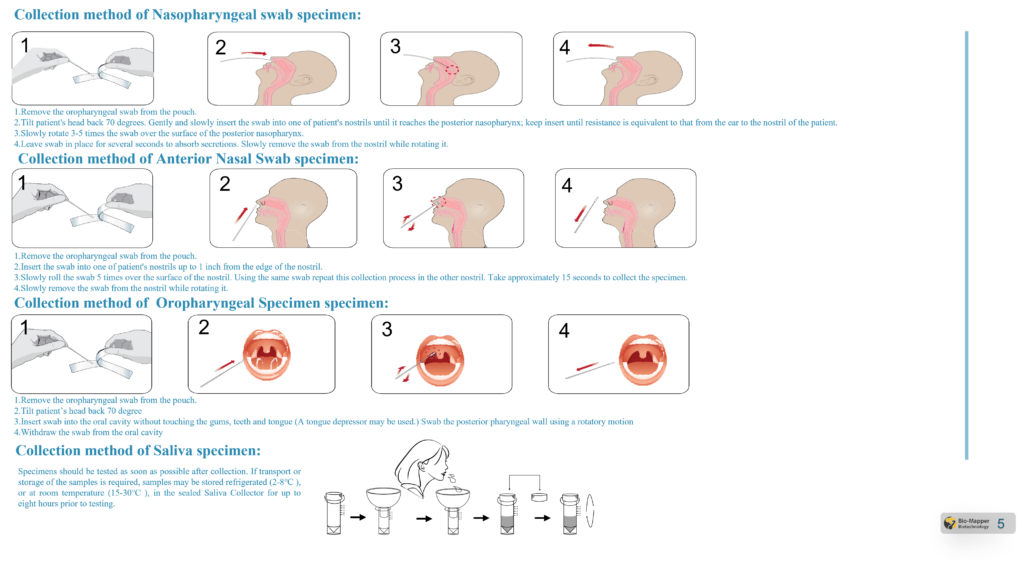
Specimen Requirement
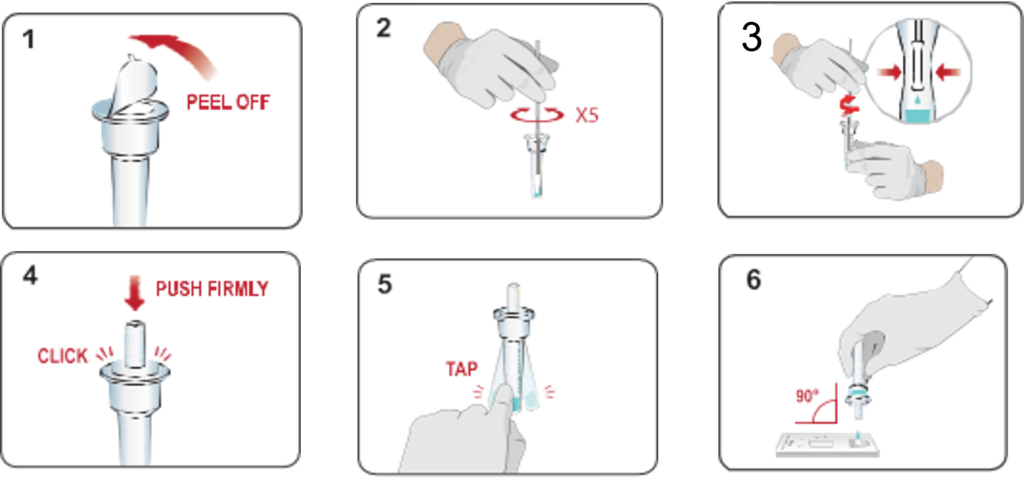
Test Procedure
Swab
1.peel off aluminum foil seal from the top of the extraction vial containing the extraction buffer.
2.Place the swab into the extraction vial. Rotate the swab vigorously at least 5 times.
3.Remove the swab by rotating against the extraction vial while squeezing the sides of the vial to release the liquid from the swab. Properly discard the swab.
4.Close the vial by pushing the cap firmly onto the vial.
5.Mix thoroughly by flicking the bottom of the tube.
6.Invert the extraction vial and hold the sample vertically above the sample well. Squeeze the vial gently.Allow 2-3 drops of sample to fall into the sample well.
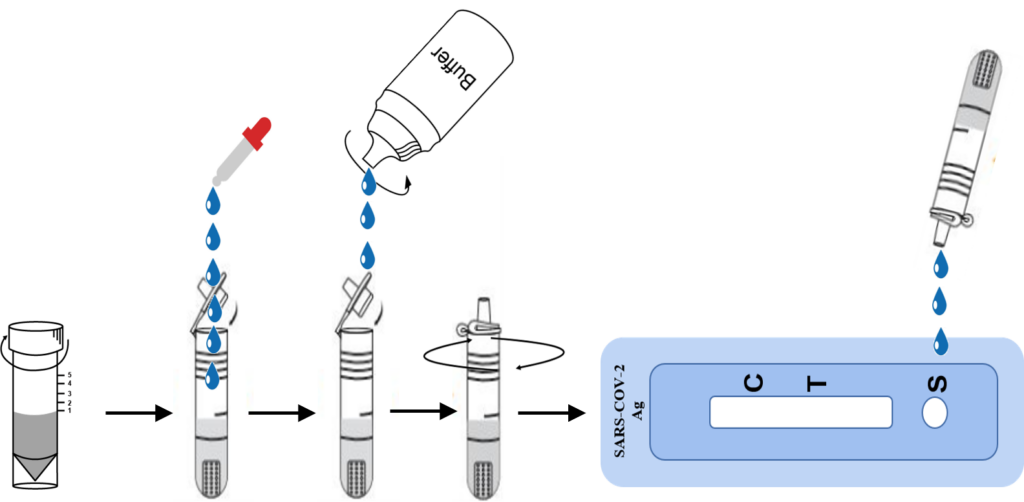
Saliva
Saliva
1.Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. For best results, the assay should be performed within one hour.
2.Add 100ul (3 drops) of the Extraction Solution into the Extraction tube. Add 200ul (6 drops) the patient saliva(saliva collector tube) specimen into the Extraction Tube.
3.Gently mix Extraction reagent solution. To be sure the saliva and extraction buffer mix well.
4.Put on the tube tip, then add 3 drops(100ul) of extracted sample into the sample well. Do not handle or move the Test Device until the test is complete and ready for reading.
Interpretation of Results
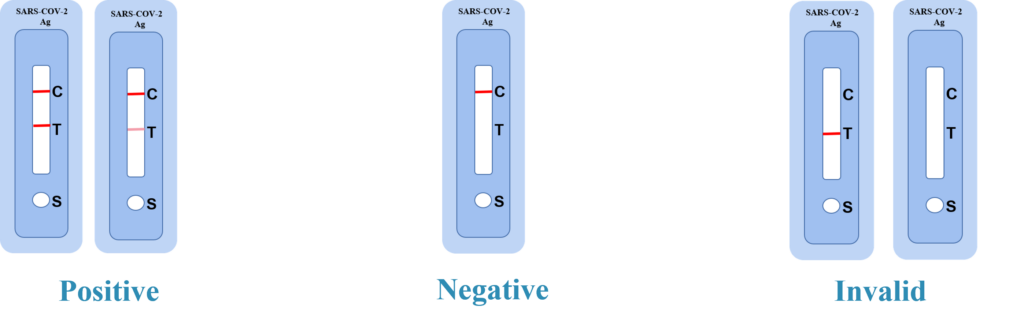
Positive: Two distinct red lines appear. One line should be in the control region(C) and the other line should be in the test region(T).
Negative: One red line appears in the control region(C).No red line appears in the test region(T).The negative result does not indicate the absence of analytes in the sample, it only indicates the level of tested analytes in the sample is less than cut-off level.
INVALID: No colored lines appear, or control line fails to appear, indicating that the operator error or reagent failure. Verify the test procedure and repeat the test with a new testing device.
Performance Characteristics
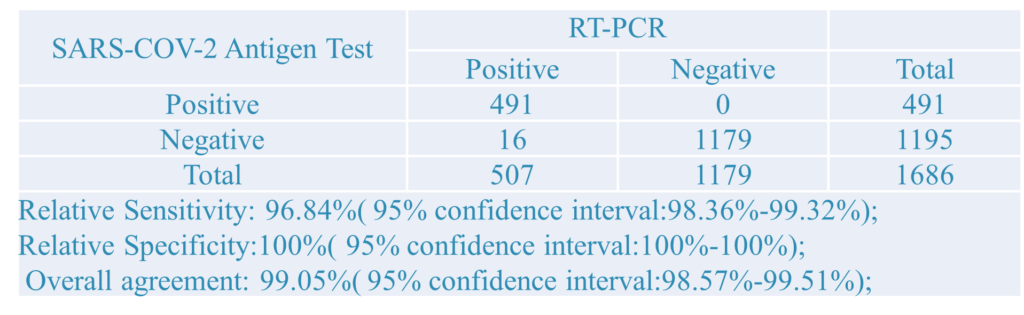
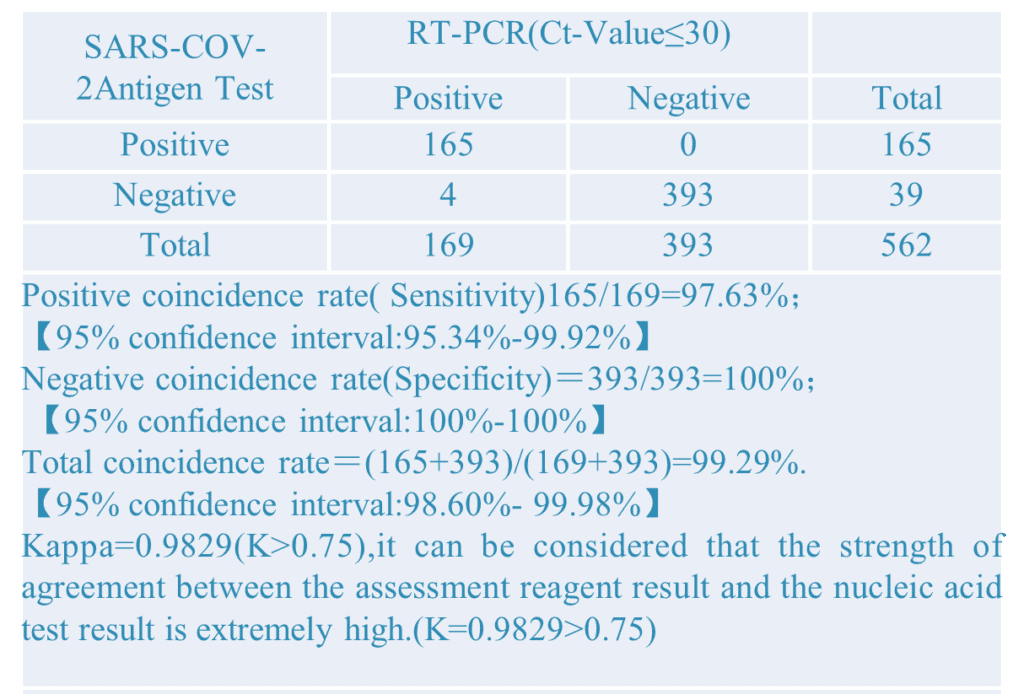
1.Clinical Results:A total of 1686 samples from susceptible subjects were test by the PCR test and CT Detection from three hospitals. Comparison for all subjects is showed in the following table:
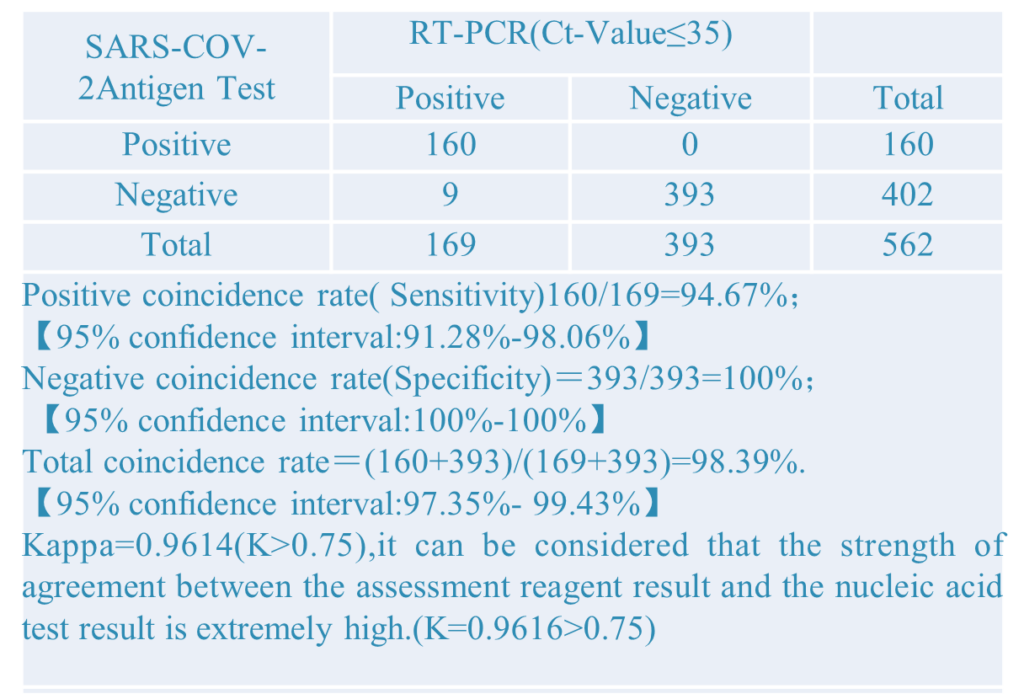
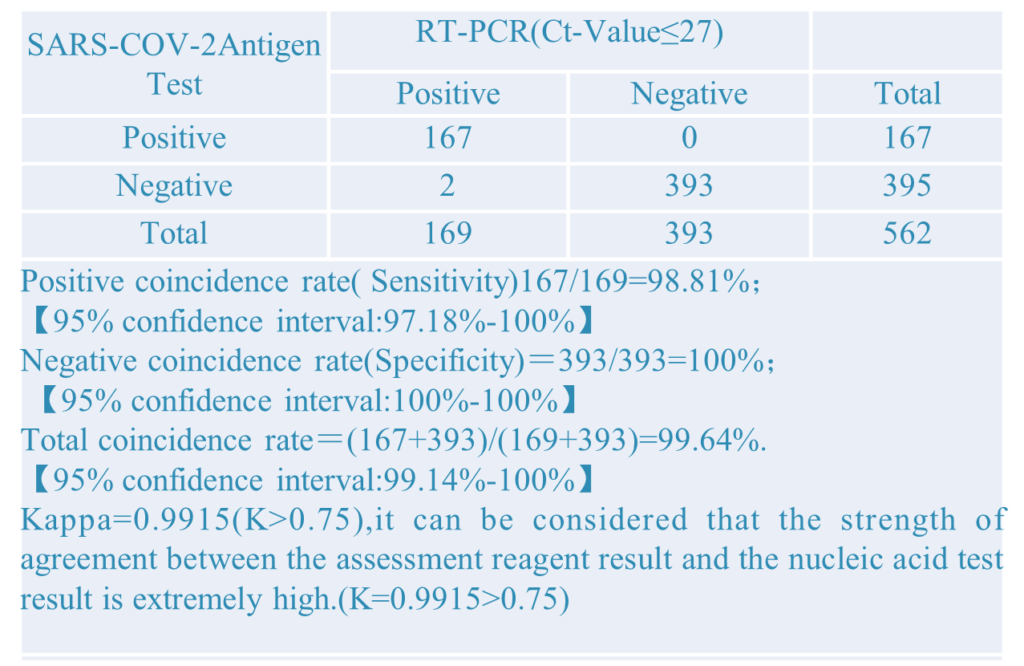
2.Different PCR CT-Value: Setting different PCR CT-value to did the comparative verification experiment. The results as below:
3.Conclusion:
The above results show that the sensitivity of the kit is 96.84%, the specificity is 100%, and the total coincidence rate is more than 99.05%, which is consistent with the clinical diagnosis results and has strong clinical application value. It can assist nucleic acid detection and SARS-CoV-2 IgG/IgM detection in clinical applications.
Limit Detection
1.Specimen to be tested:
Limit detection reference:
A:Recombinant Antigen:RS1(1000pg),R2(200pg),RS3(100pg),RS4(50pg),RS5(20pg),RS6(10pg),RS7(5pg)
B:SARS-COV-2 Culture Fluid(Heat Inacvtivated):(Order from ZeptoMetrix ,The Lot:324608 )
CS1(1.51*106 TCID50/ml),CS2(1.51*104 TCID50/ml),CS3(1.51*103 TCID50/ml),CS4(1.51*102 TCID50/ml),CS5(1.51*101 TCID50/ml),CS6(1.51*100 TCID50/ml),
Repeatable reference:R1,R2
2.Test requirement:
The detection result of the Recombinant antigen reference RS1-RS5 should all be positive,RS6 and RS7 could be negative or positive.
The detection result of the Recombinant antigen reference CS1-CS4 should all be positive,CS5 and CS6could be negative or positive.
The repeatable reference R1 and R2 should be positive and the color rendering should be uniform.
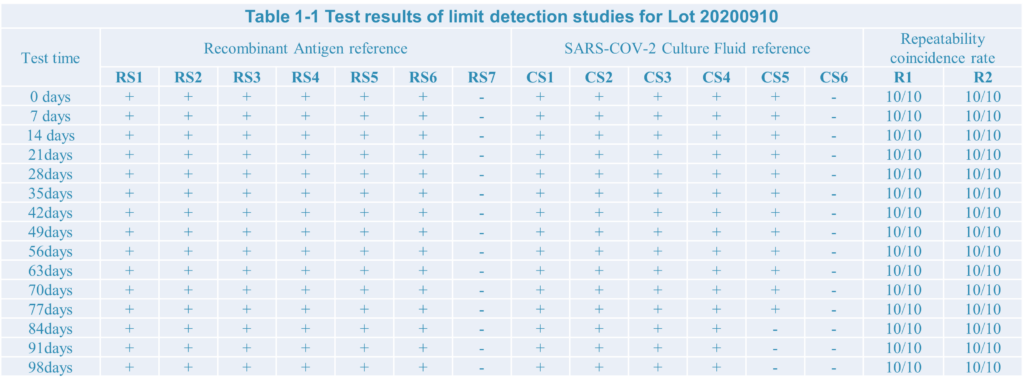
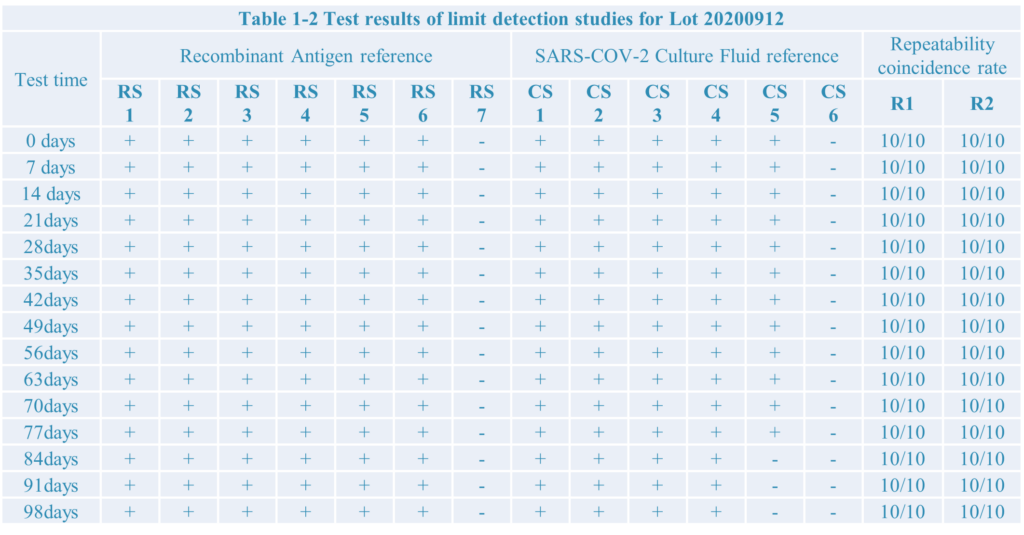
3.Three different Lot: Lot:200910;Lot:200912;Lot:200915
4.The test result:
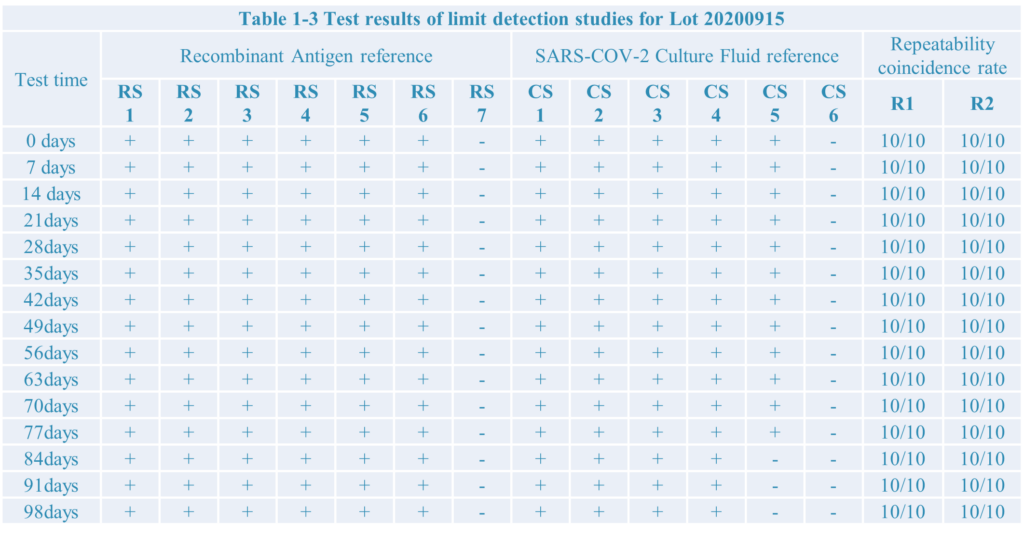
Conclusion:
After limit detection and repeat test research,the SARS-COV-2 antigen rapid test kit developed by Home test biotechnology Co.,Ltd have a good performance.
During testing the recombiant antigen reference the limit detection is 10pg, as well as 1.51*102 TCID50/ml during testing Culture Fluid reference.
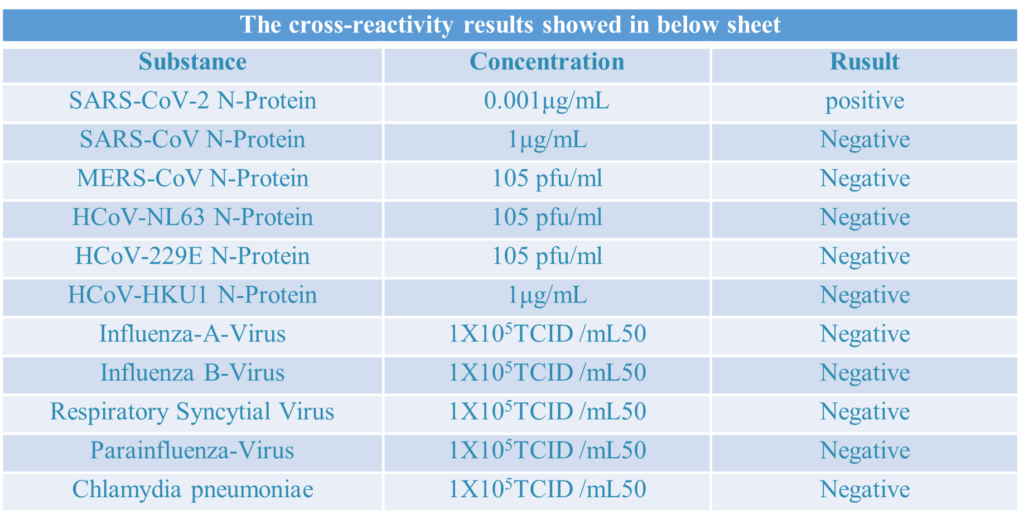
Cross-Reactivity Evaluation
Cross reactivity reference reference:
A.InfluA positive samples:5 clinical samples which clinically confirmed,2 recombinant antigens(1ug/ml) from different sources, 1 culture sample(1*10 5 TCID50/ml).
B.InfluB positive samples:2 clinical samples which clinically confirmed,2 recombinant antigens(1ug/ml) from different sources, 1 culture sample(1*10 5 TCID50/ml).
C.RSV positive samples:2 clinical samples which clinically confirmed,2 recombinant antigens(1ug/ml) from different sources, 1 culture sample(1*10 5 TCID50/ml).
D.SARS positive samples:3 recombinant antigens(1ug/ml) from different sources.
E.MERS-CoV N-Protein positive samples:4 recombinant antigens(105 pfu/ml) from different sources.
F.ParaFlu positive samples:1 clinical samples which clinically confirmed,1 culture sample(1*10 5 TCID50/ml).
G.HCoV-NL63-N-Protein positive samples: 4 recombinant antigens(105 pfu/ml) from different sources.
H.HCoV-229E-N-Protein positive samples:2 recombinant antigens(105 pfu/ml) from different sources.
I.HCoV-HKU1-N-Protein positive sample:2 recombinant antigens(105 pfu/ml) from different sources.
J.Ch1amydia pneumoniae psotive sample: 1 culture sample(1*10 5 TCID50/ml).
K: Negative serum/plasma samples:20 samples of SARS-COV-2 negative serum/plasma

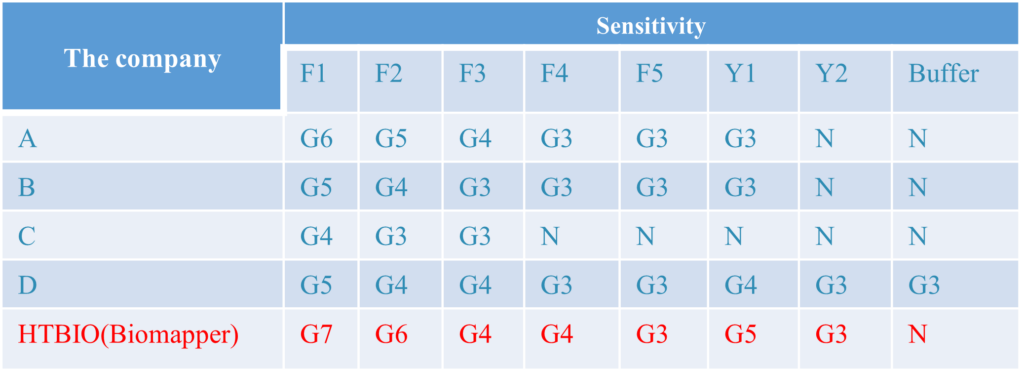
Detection of Different Mutants
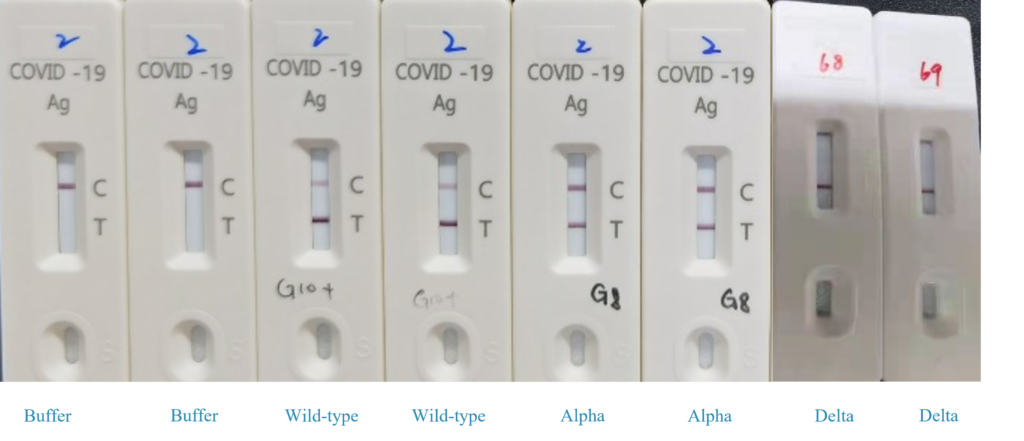
Conclusion:
We use our uncut sheet to test the mutants , our uncut sheets can be detected the Alpha, Delta mutants. Whether detection the Lambda mutant, under testing.