SARS-COV-2 Raw Materials
The 2019 novel coronavirus, on January 12, 2020, the World Health Organization officially named it 2019-nCovid. Coronaviruses are a large family of viruses that are known to cause colds and more serious diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The new coronavirus is a new strain of coronavirus that has never been found in the human body before.
On January 15, 2021, a variant of the new coronavirus from Brazil has been discovered in the UK. On February 21, according to a report from Russia Today, Indian health officials stated that as many as 240 new variants of the new coronavirus strain have been discovered in various parts of India, and active countermeasures are needed.
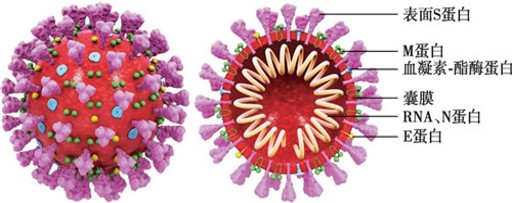
The 2019 Novel Coronavirus (SARS-COV-2) is a type of virus that is spherical in shape, has protrusions on its surface, and looks like a “crown” under electron microscope. SARS-COV-2 is composed of protein and RNA, and its membrane surface is mainly composed of three structural proteins: Spike Protein (S), Envelope Protein (E) and Membrane Protein (M) ; Inside the virus is RNA, the nucleic acid material responsible for virus reproduction, which is wrapped and protected by nucleoprotein (N).
Raw materials for SARS-COV-2 Antigen Test
SARS-COV-2 antigen detection is based on the immunological reaction of antigen and antibody to detect the presence of new coronavirus in the human body. The types of test samples currently approved in the market include: nasopharyngeal swabs, saliva, sputum, oral secretions, blood, etc. With the optimization of the performance of raw materials and product processes, the specificity and sensitivity of antigen detection reagents are getting higher and higher, which can be comparable to nucleic acid detection reagents. However, the rapid detection reagents for antigens are convenient to operate, fast for detection, and do not require professional operators and equipment. Therefore, in some occasions, especially the opening of home self-tests, rapid antigen detection reagents can be effective as a new covid-19 diagnosis and screening. tool. The SARS-COV-2 NP monoclonal antibody and SARS-COV-2 S-RBD monoclonal antibody developed and produced by Bio-mapper can be used in the development and production of antigen detection reagents. All recommended pairs have been tested on clinically positive samples, virus lysates and inactivated vaccines, and have shown very good performance in different test samples. Of course, the antibody screening work is not over, and Mai Yue Biological will continue to screen new antibody pairs for verification and continue to maintain communication with customers, keeping an eye on clinical changes at any time.
| SARS-CoV-2 Antigen Rapid Test Kit | |||
|---|---|---|---|
| PCR Results | Positive | Negative | Total |
| Positive | 491 | 0 | 491 |
| Negative | 16 | 1179 | 1195 |
| Total | 507 | 1179 | 1686 |
| Relative Sensitivity: 96.84%( 95% confidence interval:98.36%-99.32%); Relative Specificity:100%( 95% confidence interval:100%-100%); Overall agreement: 99.05%( 95% confidence interval:98.57%-99.51%); |
|||
Raw materials for SARS-COV-2 Antigen Test
Test sample type:nasopharyngeal swabs, saliva, sputum, oral swab,Anterior nasal swab
The profermance:Sensitivity>90%;Specificity>95%
Detection Limit:Recombinant Antigen:<10pg
Virus culture:dilution 10000 times
Inactivated virus:1.51 ˟10 TCID50/ml
Applicable method:Double antibody sandwich method, biotin amplification system Applicable platform:Latex, colloidal gold
| Product Name | Cat | Host | Recommended use | Epitope | Test sample type |
|---|---|---|---|---|---|
| SARS-COV-2 NP Antibody | BMGMC19N11 | Mouse | coating | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N21 | Mouse | conjugation | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N22 | Mouse | coating | N | Swab/saliva/sputum |
| SARS-COV-2 NP Antibody | BMGMC19N31 | Mouse | conjugation | N | Swab/saliva/sputum |
| SARS-COV-2 NP Antibody | BMGMC19N32 | Mouse | coating | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N41 | Mouse | conjugation | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N42 | Mouse | coating | N | Swab/saliva/sputum |
| SARS-COV-2 NP Antibody | BMGMC19N51 | Mouse | conjugation | N | Swab/saliva/sputum |
| SARS-COV-2 NP Antibody | BMGMC19N52 | Mouse | coating | N | Swab/saliva/sputum |
| SARS-COV-2 NP Antibody | BMGMC19N53 | Mouse | conjugation | N | Swab/saliva/sputum |
| SARS-COV-2 NP Antibody | BMGMC19N61 | Mouse | coating | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N62 | Mouse | conjugation | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N71 | Mouse | coating | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N72 | Mouse | conjugation | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N81 | Mouse | coating | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N82 | Mouse | conjugation | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N91 | Mouse | coating | N | Swab |
| SARS-COV-2 NP Antibody | BMGMC19N92 | Mouse | conjugation | N | Swab |
| SARS-COV-2 RBD Antibody | BMGMC19S11 | Mouse | coating | S-RBD | Swab |
| SARS-COV-2 RBD Antibody | BMGMC19S12 | Ecoli | coating | S-RBD | Swab |
| SARS-COV-2 RBD Antibody | BMGMC19S13 | Ecoli | conjugation | S-RBD | Swab |
| Goat anti-mouse IgG | BMGCT11 | Goat | coating | / | Control lines |
| Mouse IgG | BMGCT12 | Mouse | conjugation | / | Control lines |
| Goat anti-Rabbit IgG | BMGCT21 | Goat | coating | / | Control lines |
| Rabbit IgG | BMGCT22 | Rabbit | conjugation | / | Control lines |
| Goat anti-chicken IgY | BMGCT31 | Goat | coating | / | Control lines |
| chicken IgY | BMGCT32 | Chicken | conjugation | / | Control lines |
| Blocking Antibody | BMGBL01 | Mouse | / | / | / |
| Blocking Antibody | BMGBL02 | Mouse | / | / | / |
Raw materials for SARS-COV-2 Antibody Test
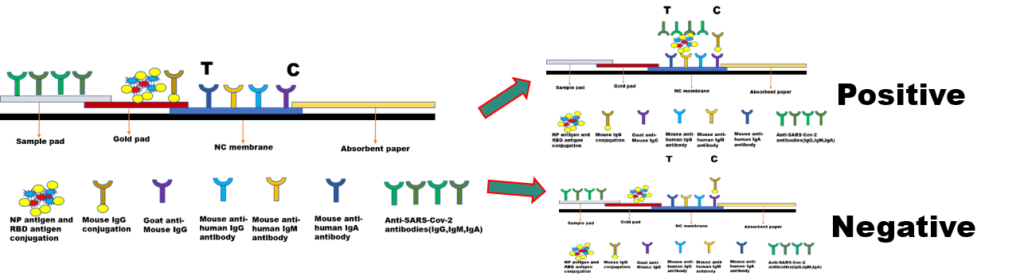
After SARS-COV-2 virus enters the human body, B cells will produce different types of antibodies. In the early stage of infection (7 days of initial illness), the human body will produce IgA and IgM antibodies, but they can only survive in the body for about 30 days. IgG antibodies are produced late, but they can stay in the body for several months. At the same time, the titers of IgG antibodies in the recovery phase are several times higher than the gradient in the acute infection phase. Therefore, the detection of SARS-CoV-2 IgG/IgM (IgA) can be used for disease course monitoring, epidemiological investigation, and large-scale preliminary screening. For the detection of different types of antibodies, the process and stage of the disease can be distinguished. The N protein (prokaryotic expression), the S1 protein (eukaryotic expression), the RBD protein (eukaryotic expression), mouse anti-human IgG monoclonal antibody, mouse anti-human IgM monoclonal antibody, mouse Anti-human IgA monoclonal antibody can be used for SARS-COV-2 IgG, IgM, IgA antibody typing detection, with high sensitivity and high specificity. Good antigen/antibody can greatly improve the accuracy of detection reagents. As the virus continues to mutate, Biomapper has designed and developed antigens for different immune strains to respond to different mutant strains at any time. At the same time, Biomapper has also developed two sufficient proteins (N protein and S protein) for the positive quality control of new crown antigen detection.
| Product Name | Cat | Host | Recommended use | Epitope | Test sample type |
|---|---|---|---|---|---|
| SARS-COV-2 NP Antigen- Wild Strain | BMGNW11 | Ecoli | Conjugation | N | Whole blood/serum/plasma |
| SARS-COV-2 NP Antigen-Delta Mutant Strain | BMGND21 | Ecoli | Conjugation | N | Whole blood/serum/plasma |
| SARS-COV-2 NP Antigen--B.1.1.7 Mutant Strain | BMGNB31 | Ecoli | Conjugation | N | Whole blood/serum/plasma |
| SARS-COV-2 NP Antigen--Lambda Mutant Strain | BMGNL41 | Ecoli | Conjugation | N | Whole blood/serum/plasma |
| SARS-COV-2 S1 Antigen | BMGSS12 | HEK293 | Conjugation | S1 | Whole blood/serum/plasma |
| SARS-COV-2 RBD Antigen | BMGSRW2 | HEK293 | Conjugation | RBD | Whole blood/serum/plasma |
| SARS-COV-2 RBD Antigen-Delta Mutant Strain | BMGSRD2 | HEK293 | Conjugation | RBD | Whole blood/serum/plasma |
| SARS-COV-2 RBD Antigen-Lambda Mutant Strain | BMGSRL2 | HEK293 | Conjugation | RBD | Whole blood/serum/plasma |
| Mouse anti-human IgG antibody | BMGGC01 | Mouse | coating | IgG | Whole blood/serum/plasma |
| Mouse anti-human IgM antibody | BMGMC01 | Mouse | coating | IgM | Whole blood/serum/plasma |
| Mouse anti-human IgA antibody | BMGGA01 | Mouse | coating | IgA | Whole blood/serum/plasma |
| Blocking antibody | BMGBL01 | Mous | Sample pad Treatment | - | Whole blood/serum/plasma |
| Blocking antibody | BMGBL02 | Mouse | Sample pad Treatment | - | Whole blood/serum/plasma |
| RBC antibody | BMGRB01 | Mouse | Sample pad Treatment | - | Whole blood/serum/plasma |
| Goat anti-mouse IgG | BMGCT11 | Goat | coating | - | Whole blood/serum/plasma |
| Mouse IgG | BMGCT12 | Mouse | conjugation | - | Whole blood/serum/plasma |
| Goat anti-Rabbit IgG | BMGCT21 | Goat | coating | - | Whole blood/serum/plasma |
| Rabbit IgG | BMGCT22 | Rabbit | conjugation | - | Whole blood/serum/plasma |
| Goat anti-chicken IgY | BMGCT31 | Goat | coating | - | Whole blood/serum/plasma |
| Chicken IgY | BMGCT32 | Chicken | conjugation | - | Whole blood/serum/plasma |
Raw materials for Neutralizing Antibdy test
After the human body is vaccinated, it will produce a protective antibody to deal with the virus that enters the human body again. This protective antibody is called a neutralizing antibody. The virus surface protein is the key(RBD protein) for the virus to enter the cell. It can open the “lock” of the cell receptor protein (ACE2 protein) to enter the cell and initiate its replication process. The body’s protective antibody response is precisely by recognizing and blocking the combination of this “key” and “lock” to block the virus from entering the cell. Now the key target of vaccine development is the “key” to the covid-19 virus. How to monitor the effect of the vaccine after vaccination will become a new demand point. How to grasp effective, scientific, and truly protective antibodies will be the most important key point of this product. The ACE2 protein is an important gateway for the virus to enter the human body. It is based on the structural binding between the ACE2 protein and the RBD protein, and the specific binding between the RBD antibody and the RBD protein. At the same time, the current vaccine targets are all against the RBD protein, ACE protein Competitively binding to RBD protein with RBD antibody will be an effective way to detect neutralizing antibody. The ACE2 protein and RBD protein, which are closer to the natural structure, are the prerequisite for the efficient combination of these two proteins. The ACE2 protein and RBD protein developed and produced by Mai Yue Biological are expressed and purified in HEK293 cells, and they are closer to the natural structure. The combination of the two is more in line with the structural combination of the lock and key of the natural virus. At the same time, the RBD monoclonal antibodies, RBD polyclonal antibodies and RBD recombinant antibodies screened by the company can be used as a positive quality control for the neutralizing antibody project. Provide effective tools for the development of the project.
| NP Antibody | S1/RBD Antibody | Result |
|---|---|---|
| Positive | Positive | The vaccine has an immune effect |
| Negative | Positive | Vaccination of mRNA vaccine, adenovirus vaccine, inactivated vaccine is effective |
| Positive | Negative | Inactivated vaccines have an immune effect |
| Negative | Negative | The vaccination time is short or the vaccine does not produce immunity |
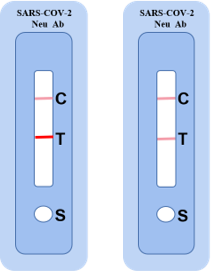
SARS-COV-2 Neutralizing antibody test-Competition Inhibition method
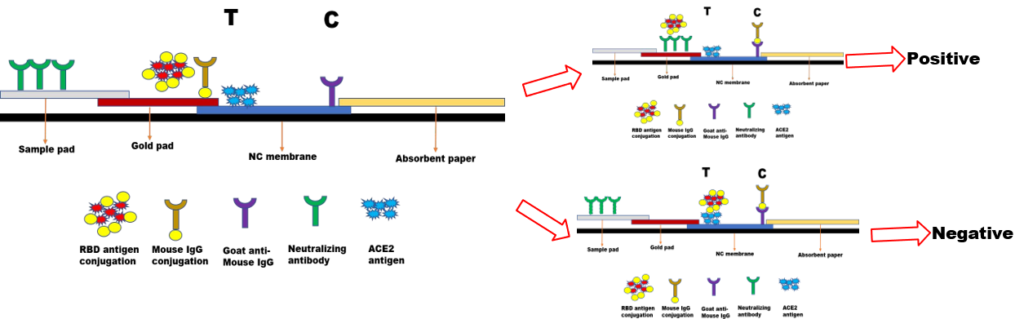
| Product Name | Cat | Host | Recommended use | Epitope | Test sample type |
|---|---|---|---|---|---|
| SARS-COV-2 ACE2 Antigen | BMGNAE11 | HEK293 | Coating | ACE2 | WB/S/P |
| SARS-COV-2 ACE2-FCAntigen | BMGNAE12 | HEK293 | Coating | ACE2 | WB/S/P |
| SARS-COV-2 RBD Antigen | BMGSRW2 | HEK293 | Conjugation | RBD | WB/S/P |
| SARS-COV-2 Spike-RBD-Fc Antigen | BMGSRWC3 | HEK293 | Conjugation | RBD | WB/S/P |
| SARS-COV-2 RBD Antigen | BMGSRWG2 | HEK293 | Conjugation | RBD | WB/S/P |
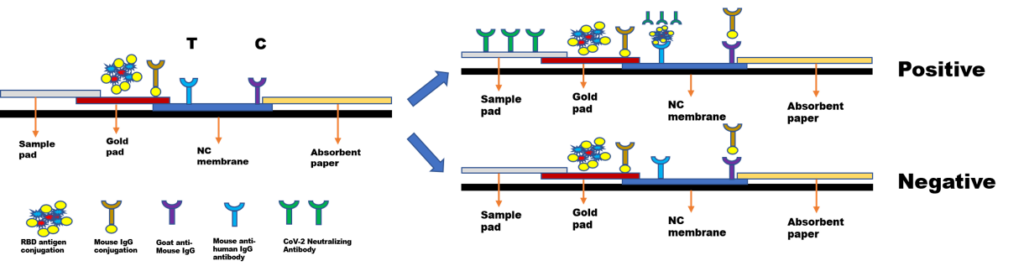
Raw materials for RBD IgG Antibdy test(Capture method)
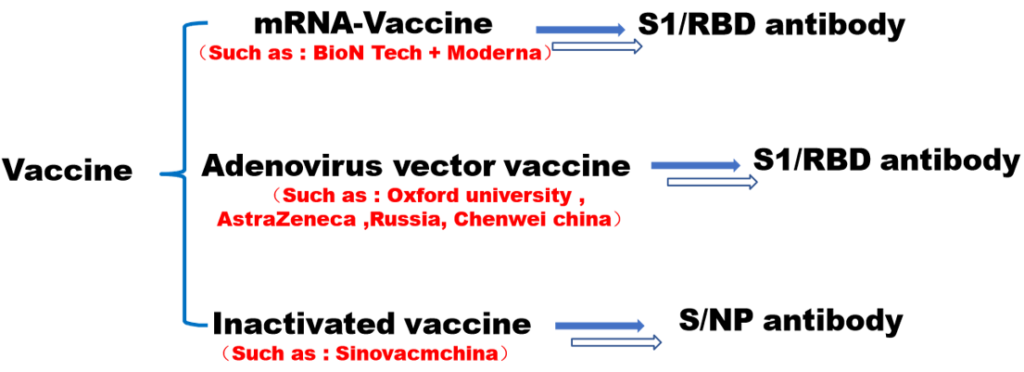
There have three kinds of vaccine.mRNA vaccine, adenovirus vector vaccine and Inactivated vaccine. All vaccine have the RBD epitopes which are able elicit neutralizing antibodies in SARS-COV-2 recent stduies. So detecting the RBD IgG antibody will help us to study whether vaccine activity.The test cassette consists of: 1)a burgundy colored conjugate pad containing SARS-COV-2 S-RBD antigen conjugated with colloid gold (SARS CoV-2 conjugates) and mouse IgG-gold conjugates, 2) a nitrocellulose membrane strip containing a test band (T band) and a control band (C band). The test band is pre-coated with Mouse anti-human IgG antibody for capture bind to RBD with neutralizing antibody , in order to detection the RBD antibody in serum, plasma and whole blood. The C band is pre-coated with goat anti mouse IgG.
When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. If there is antibody producing after vaccine, the RBD antibody will be conjugated with RBD antigen, than the Mouse anti-human IgG antibody can bind to RBD antigen with Neutralizing Antibody. So the test line will be colored. If no antibody produced, the RBD antigen will not bind to Mouse anti-human IgG antibody , the test line will not be colored. These combo cassette can distinguish the source of the antibody, which is very important for detecting the effect of the vaccine.
| Product Name | Cat | Host | Usage | Epitope | Test sample type |
|---|---|---|---|---|---|
| SARS-COV-2 RBD Antigen | BMGSRW2 | HEK293 | Conjugation | RBD | WB/S/P |
| SARS-COV-2 Spike-RBD-Fc Antigen | BMGSRWC3 | HEK293 | Conjugation | RBD | WB/S/P |
| SARS-COV-2 RBD Antigen | BMGSRWG2 | HEK293 | Conjugation | RBD | WB/S/P |
| SARS-COV-2 RBD Antigen | BMGSRWG3 | HEK293 | Conjugation | S-RBD | WB/S/P |
| Mouse anti-human IgG antibody | BMGGC01 | Mouse | Coating | IgG | WB/S/P |
| Blocking antibody | BMGBL01 | Mous | Sample pad Treatment | - | WB/S/P |
| Blocking antibody | BMGBL02 | Mouse | Sample pad Treatment | - | WB/S/P |
| RBC antibody | BMGRB01 | Mouse | Sample pad Treatment | - | WB/S/P |
| Goat anti-mouse IgG | BMGCT11 | Goat | coating | - | WB/S/P |
| Mouse IgG | BMGCT12 | Mouse | conjugation | - | WB/S/P |
| Goat anti-Rabbit IgG | BMGCT21 | Goat | coating | - | WB/S/P |
| Rabbit IgG | BMGCT22 | Rabbit | conjugation | - | WB/S/P |
| Goat anti-chicken IgY | BMGCT31 | Goat | coating | - | WB/S/P |
| Chicken IgY | BMGCT32 | Chicken | conjugation | - | WB/S/P |